- 泽任订制
- FST动物实验器械
- 瑞士ideal-tek镊子
- 瑞士Dumont镊子
- Weller
- Techni-Tool
- 瑞士Rubis镊子
- arosurgica
- Genesee Scientific
- Gilder Grids
-
Entegris
- WEICON
- ACCURIS
- AIMS
- RMC钻石刀
- Sipel镊子
- 德国WIHA
-
Si-Mat
- Jokari
- Circuitmedic
-
Glissando病理切片扫描仪
- KNIPEX(凯尼派克)
- Swann-morton
- Cellpoint Scientific
- Swanstrom
- All-spec
- 新华器械
- Excelta
- MENDA
- MEISEI
- Erem
- Regine
-
OPTRASCAN病理扫描仪
- Benchmark
-
IDEAL
- 大龙仪器
- 五洋医疗
- Hozan(宝山)
- 3M
- Stoelting
- Roboz
-
Diatome钻石刀
- Fine Science Tools
- PLATO
- NanoSoft
-
Avantama
-
PSI打击器及配件
- Aptum
- xuron
- SPI
-
抗原检测
- ASI Instruments
-
上海一恒
-
上海雷磁
- Quantifoil
- 上海方需
- 苏州六六
-
Hamilton
- ISMART
-
显微镜
-
Bonllmann
- AVEN
- 塔望科技
-
瑞典Haglof激光测距仪
- STILLE
-
美国安维迪
- 德国贝朗蛇牌
- Medline
-
蛋白纯化系统
-
OHAUS奥豪斯
- 康博特森
- Ossila
- 赛宁(苏州)生物
- Bernstein
- 洁盟清洗机
- Ted Pella
- DSI
- Keller
-
Smart Tweezers
- 瑞士PB
-
Microscopes Internat...
- Lattice Gear
-
凯氏定氮仪
- lindstrom
- 美国EMS
-
1-material
-
仪器
- realisticflies
- Boive
- Zivic
Luminosyn™ DPP-DTT
Luminosyn™ DPP-DTT (also referred to as PDPP2T-TT-OD) is now available.
High molecular weight
Higher molecular weight offers higher charge mobility
High purity
DPP-DTT is purified via Soxhlet extraction with methanol, hexane and chlorobenzene under an argon atmosphere
Batch-specific GPC data
Have confidence in what you are ordering; batch-specific GPC data for your thesis or publications
Large quantity orders
Plan your experiments with confidence with polymers from the same batch
OFET and Sensing Applications
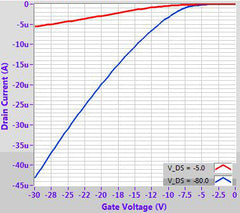
The exceptional high mobility of this polymer of up to 10 cm2/Vs [2] via solution-processed techniques, combined with its intrinsic air stability (even during annealing) has made PDPP2T-TT-OD of significant interest for OFET and sensing purposes.
While the highest mobilities require exceptional molecular weights of around 500 kD (and with commensurate solubility issues), high mobilities in the region of 1-3 cm2/Vs can still be achieved with good solution-processing at around 250 kD. As such, we have made a range of molecular weights available to allow for different processing techniques.
In our own tests, we have found that by using simple spin-coating onto an OTS-treated silicon substrate (using our prefabricated test chips), high mobilities comparable to the literature can be achieved (1-3 cm2/Vs). Further improvements may also be possible with more advanced strain-inducing deposition techniques.



Photovoltaic Applications
Although shown as a promising hole-mobility polymer for OFETs, when used as the donor material in a bulk heterojunction photovoltaic (with PC70BM as the acceptor), initial efficiencies of 1.6% were achieved for DPP-DTT [3]. The low device metrics were attributed to poor film morphology. However, a higher efficiency of 6.9% was achieved by using thicker film (220 nm) [4].
PDPP2T-TT-OD has also recently been used successfully as an active-layer dopant material in PTB7-based devices [5]. An improvement in device performance was observed, with average efficiencies increasing from 7.6% to 8.3% when the dopant concentration of DPP-DTT was 1 wt%. The use of DPP-DTT as a high-mobility hole-interface layer for perovskite hybrid devices has also been investigated [6].
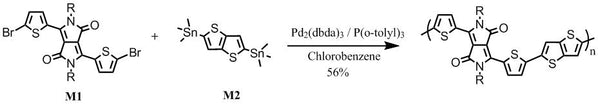
Synthetic route
DPP-DTT synthesis: DPP-DTT was synthesised by following the procedures described in [2] and [3] (please refer to the following references):
With 2-thiophenecarbonitrile and dimethyl succinate as starting materials in t-amyl alcohol, it gave 3,6-Dithiophen-2-yl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione. Alkylation of 3,6-Dithiophen-2-yl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione with 2-octyldodecylbromide in dimethylformamide afforded 3,6-bis(thiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione. Further bromination gave 3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione (M1).

Further reaction of M1 with 2,5-bis(trimethylstannyl)thieno[3,2-b]thiophene (M2) under Stille coupling conditions gave the target polymer DPP-DTT, which was further purified via Soxhlet extraction with methanol, hexane and then chloroform.
General Information